Please read and accept terms before proceeding
This website and the information contained herein are not intended for, and must not be accessed by, or distributed or disseminated to, persons resident or physically present in Australia, South-Africa, Hong Kong, Japan, Canada or the United States of America, and this website does not constitute an offer to sell or the solicitation of an offer to buy or acquire, any shares, rights or other securities of the Company in Australia, South-Africa, Hong Kong, Japan, Canada or the United States or in any other country where it would be prohibited by local laws or other regulations.
The shares, rights or other securities of the Company referred to on this website have not been and will not be registered under the U.S. Securities Act of 1933, as amended, and may not be offered or sold in the United States absent registration or an applicable exemption from registration thereunder.
Access to the information and documents contained on the following websites may be illegal in certain jurisdictions, and only certain categories of persons may be authorized to access such information and documents. All persons residing outside of Australia, South-Africa, Hong Kong, Japan, Canada or the United States who wish to have access to the documents contained on this website should first ensure that they are not subject to local laws or regulations that prohibit or restrict their right to access this website, or require registration or approval for any acquisition of securities by them. The Company assumes no responsibility if there is a violation of applicable law and regulations by any person.
By clicking the link below in order to see the available documents you are considered to certify that:
- You are a resident and physically present outside Australia, South-Africa, Hong Kong, Japan, Canada or the United States;
- You are not a resident or physically present in any of the Member States of the European Economic Area (other than Finland or Sweden) having implemented the Directive 2003/71/EC of 4 November 2003 on the prospectus to be published when securities are offered to the public or admitted to trading and amending Directive 2001/34/EC (as amended) ("Prospectus Directive"), unless an exemption in the Prospectus Directive is applicable; and
- You are a resident and physically present (a) in Finland or Sweden or (b) outside Finland and Sweden and each of the jurisdictions referred to in clauses (1) through (2) above and, in that case, are authorized to access the information and documents on this website without being subject to any legal restriction and without any further action required by the Company.
By clicking the link below I confirm that I have read, understood and agree to comply with all restrictions set forth above:
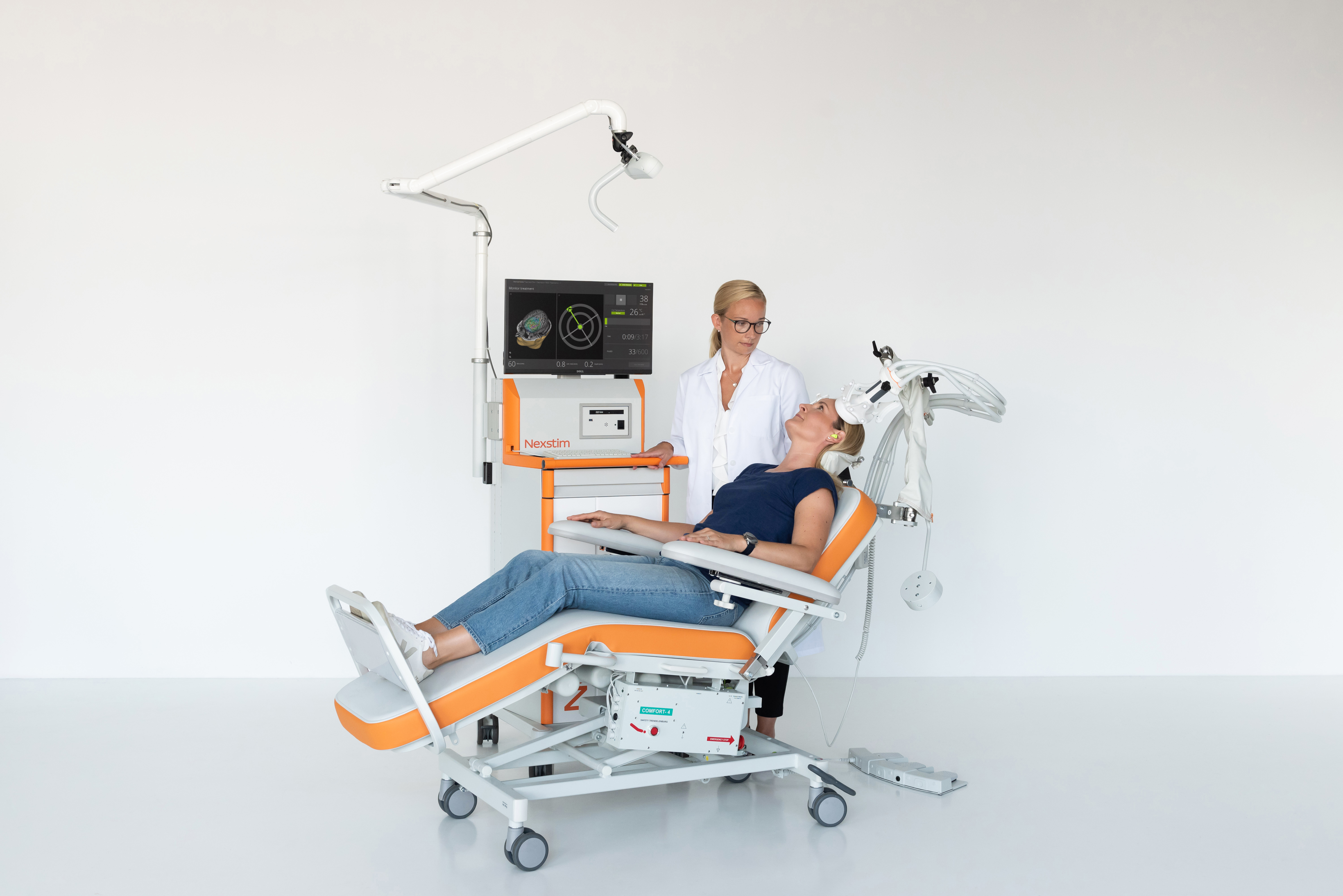
Learn more about NBS 6 for Depression Therapy
Indications for use
Depression
Nexstim's noninvasive NBS 6 is cleared by the Food and Drug Administration (FDA, K171902 & K182700). Nexstim NBS 6 is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode.
CE mark: Nexstim NBS 6 for depression is intended to be used for treatment of major depressive disorder (MDD) by targeting and delivering noninvasive repetitive TMS stimulation to the patient's dorsolateral prefrontal cortex.
Pain
The Nexstim NBS 6 is not cleared by the FDA for commercial use of the treatment of chronic pain in the United States, for investigational use only.
CE mark Intended purpose
In adult patients suffering from chronic unilateral neuropathic pain, Nexstim NBS 6 is intended to provide electric field navigated noninvasive repetitive TMS stimulation as therapy to alleviate pain. Nexstim NBS 6 is intended to be used by trained clinical professionals.
CE mark Indications for use
Nexstim NBS 6 is indicated for MRI-guided and electric field (or E-field) navigated, noninvasive repetitive TMS stimulation (rTMS) of the motor cortex as therapy to alleviate chronic unilateral neuropathic pain in adult patients. Nexstim NBS 6 is intended to be used by trained clinical professionals.
Interested in a live demo of the NBS 6?
Our team of physicians, researchers, and engineers is prepared to answer your questions. If you would like to learn more or set up a virtual demonstration for your team, please contact us at info@nexstim.com