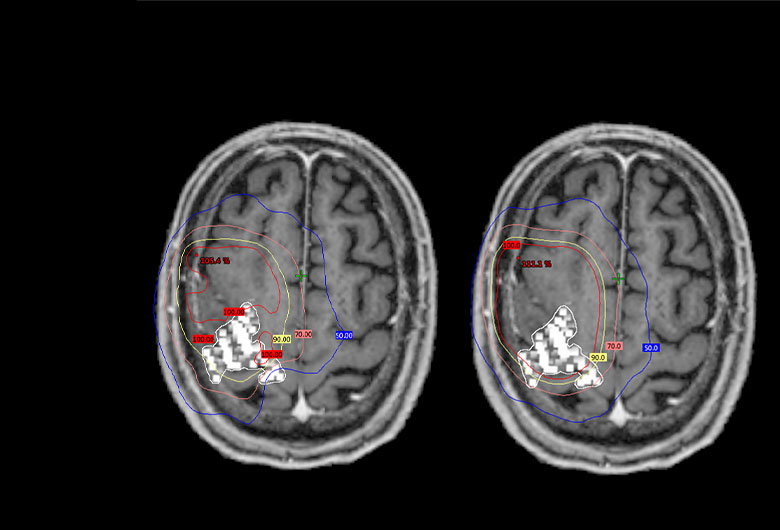
SmartFocus® nTMS Mapping Prior to Radiotherapy or -Surgery:
- For patients undergoing radiotherapy for brain metastases, nTMS defines areas in the brain responsible for motor function without surgery
- Shown to allow for significantly reduced radiation dosing to motor areas (-18.1%), without compromising treatment of the tumors. 3
- SmartFocus® nTMS mapping data can be easily and reliably integrated into GammaKnife treatment plans by the standard GammaPlan software. 4
References
1 FDA, CDRH Office of Device Evaluation K091457 Dec 2009.
2 Tarapore P, et al. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg. 2012 Aug; 117(2)
3 Schwendner, MJ. et al. The Role of Navigated Transcranial Magnetic Stimulation Motor Mapping in Adjuvant Radiotherapy Planning in Patients With Supratentorial Brain Metastases. Front Oncol. 2018 Oct 2;8:424.
4 Tokarev A.S. et al. Appliance of Navigated Transcranial Magnetic Stimulation in Radiosurgery for Brain Metastases. Journal of Clinical Neurophysiology: July 2019.
Motor Mapping
A motor-mapping session typically takes less than an hour. It is important that the patient's muscles are relaxed during the whole session, and they relieve any muscle tension. Neuronavigation of the coil and the head allows the patient to move in the chair.
1. An initial set-up process: The MRI head scan is uploaded to the software. The system creates a 3D model of the patient’s brain and then aligns the coordinate system of that 3D model to a coordinate system you give the patient sitting in the chair.
This alignment process, called registration, is a 2-part process. Part 1 involves showing the system where 3 anatomical landmarks are, both on the MRI (using a digital marker) and on the patient (using a handheld pointer called the digitizing pen). The three recommended anatomical landmarks used in Part 1 of registration are the nasion (bridge of nose) and the left and right crux of helix (specific ripple on outer ear).
Part two of registration involves using the handheld digitizing pen to show the system where specific head surface regions are, when prompted by system.
Once a patient is registered, the infrared camera will track the location of the coil in relation to the head and the user can visualize on the screen the coil and it’s e-field on the 3D model of the brain. You can see where you are stimulating and the system tracks in real time. As you move, you see everything move on the screen.
2. Measuring patients’ resting motor threshold (RMT): EMG (electromyography) surface electrodes are placed on the Abductor Pollicis Brevis (APB) muscles of the patient’s hand. Moving and activating the coil, the operator will find the area in the brain vital for controlling the thumb muscle. Next, the operator will determine how weak the stimulation intensity can be while still causing the thumb muscle to be activated, as can be seen from the EMG tracing displayed on the system screen. Knowing this intensity, the motor threshold, allows the operator to personalize the mapping to be both reliable and comfortable for the patient. Stimulation intensity is nominally set to 110% of RMT.
3. Motor mapping: EMG surface electrodes are placed on the skin over the muscles of interest. These muscles may include the foot, leg and facial muscles, as well as those for the hand, arm and shoulder. The muscles will be chosen based on the lesion's location. The operator will continue to activate the coil, moving it gradually over the head. Normally, only the areas around a tumour are mapped, so the tumour location determines which additional muscles will be monitored.
4. After the mapping: The patient's physician will normally review the results of the SmartFocus® nTMS mapping with the patient. Responses to stimuli will be shown as coloured markers in a 3D model of the brain. Taking into account other diagnostic information, the physician will discuss treatment options. SmartFocus® nTMS results can be of significant help when discussing possible trade-offs between treatment risks and expected benefits.
User-selected responses can be transferred in DICOM format for:
1) DTI-based subcortical fiber tracking,
2) Surgical navigation systems to guide surgical resection or optimize placement of grids and depth electrodes, or
3) Radiosurgery platforms to shift decrease the amount of radiation treating the eloquent tissue.
Interested in a live demo of nTMS?
Our team of physicians, researchers, and engineers is prepared to answer your questions. If you would like to learn more or set up a virtual demonstration for your team, please contact us at info@nexstim.com
Yes, I would like to know more
Indications for use
The Nexstim Navigated Brain Stimulation (NBS) System 5 is indicated for non-invasive mapping of the primary motor cortex of the brain to its cortical gyrus. The Nexstim NBS System 5 provides information that may be used in the assessment of the primary motor cortex for pre-procedural planning.
Nexstim NexSpeech®, when used together with the NBS System 5, is indicated for non-invasive localization of cortical areas that do not contain essential speech function. NexSpeech® provides information that may be used in pre-surgical planning in patients undergoing brain surgery. Intra-operatively, the localization information provided by NexSpeech® is intended to be verified by direct cortical stimulation.
The Nexstim NBS System 5 and NBS System 5 with NexSpeech® are not intended to be used during a surgical procedure.
The Nexstim NBS System 5 and NBS System 5 with NexSpeech® are intended to be used by trained clinical professionals.