Next-generation TMS, from Nexstim
To be an effective therapy, TMS needs to reach the part of your brain closely involved in emotion and mood. It’s quite small. Doctors know it by the name the left dorsolateral prefrontal cortex (DLPFC) and believe it is one of the most inter-connected parts of the brain. You can’t see it. In fact, no-one can, unless you have a brain scan.
TMS used without image-based navigation simply estimates a spot for stimulation. At Nexstim, we believe it is important that TMS targets the right location in your brain – otherwise you may not get the benefit you truly need. So, we use the latest science – 3D brain imaging and proven navigation of the TMS.
Take your life back with Nexstim
Now there is a way to get the most out of every TMS treatment. All our systems utilize SmartFocus® nTMS technology, the most advanced targeting method on the market. Every treatment is personalized to your brain’s anatomy and alertness level, to offer you:
- A measured and individualized dose of TMS
- An unrivalled level of accuracy, as demanded by brain surgeons
- Treatment sessions personalized for you and your brain
- Assurance you received your prescribed dose
- Unsurpassed safety and comfort
Hear from a patient who received SmartFocus nTMS treatment
How TMS works
TMS is an application of science that allows us to communicate directly with neurons in the brain in their own language—electricity.
Download Brochure for Patients

View
Displays 3D view of your brain from MRI scan
Measure

Personalizes the therapy to your brain state
Target

Pinpoints the therapy target in your brain
Treat

See the therapy on target
- feel confident
Repeat

Your vital data are saved - ready for your next session

SmartFocus® nTMS — a new non-invasive, non-drug therapy for depression.
Frequently Asked Questions about TMS
Here are some answers to frequently asked questions regarding nTMS treatments.
What is SmartFocus® TMS depression therapy?
SmartFocus® TMS depression therapy is a non-invasive device-based treatment, personalised just for you and your brain. As a non-drug option, TMS only influences the brain and doesn’t alter the rest of the body’s chemistry.
Transcranial Magnetic Stimulation (TMS) means stimulating the brain from outside the head using small electrical fields. To be an effective in depression therapy, this stimulation needs to reach the part of your brain closely involved in emotion and mood. Doctors know this part by the name the left dorsolateral prefrontal cortex (DLPFC). Studies have shown that in patients with depression, metabolic activity is reduced in this part of the brain.
TMS used without image-based navigation simply estimates a spot for the stimulation. At Nexstim, we know it is important that TMS targets the right location in your brain – otherwise you may not get the benefit you truly need. So, we use highly-developed 3D brain imaging model and proven navigation tools to optimize the location: without these tools the changes can’t be even seen. With our tools we can also personalise the stimulation for you.
We think focusing on you as an individual is smart. That is why we are calling our solution to SmartFocus® TMS.
Is TMS therapy same as Electro-convulsive Therapy (ECT)?
No. In electro-convulsive therapy, electric current is conducted from outside the head to inside the brain. ECT is considered as an invasive method requiring that the patient is anesthetized.
In TMS, no electric current is conducted through the scalp: Instead, the magnetic field around the TMS coil passes through the scalp and skull and generates an electric field into the brain tissue. This electric field induced in the brain is able to activate the neurons. TMS is a non-invasive therapy that doesn’t require anaesthesia. In fact, after the TMS therapy session the patient is free to continue his or her daily routine.
ECT can also have side effects, such as memory loss, which are not associated with TMS.
How does the SmartFocus® TMS depression therapy work in practice?
Every individual’s brain is different and the effects of TMS stimulation are always dependent on the state of your own brain and cortex. That is why your brain and cortex will first be measured so that you can be given a personalised SmartFocus® TMS therapy.
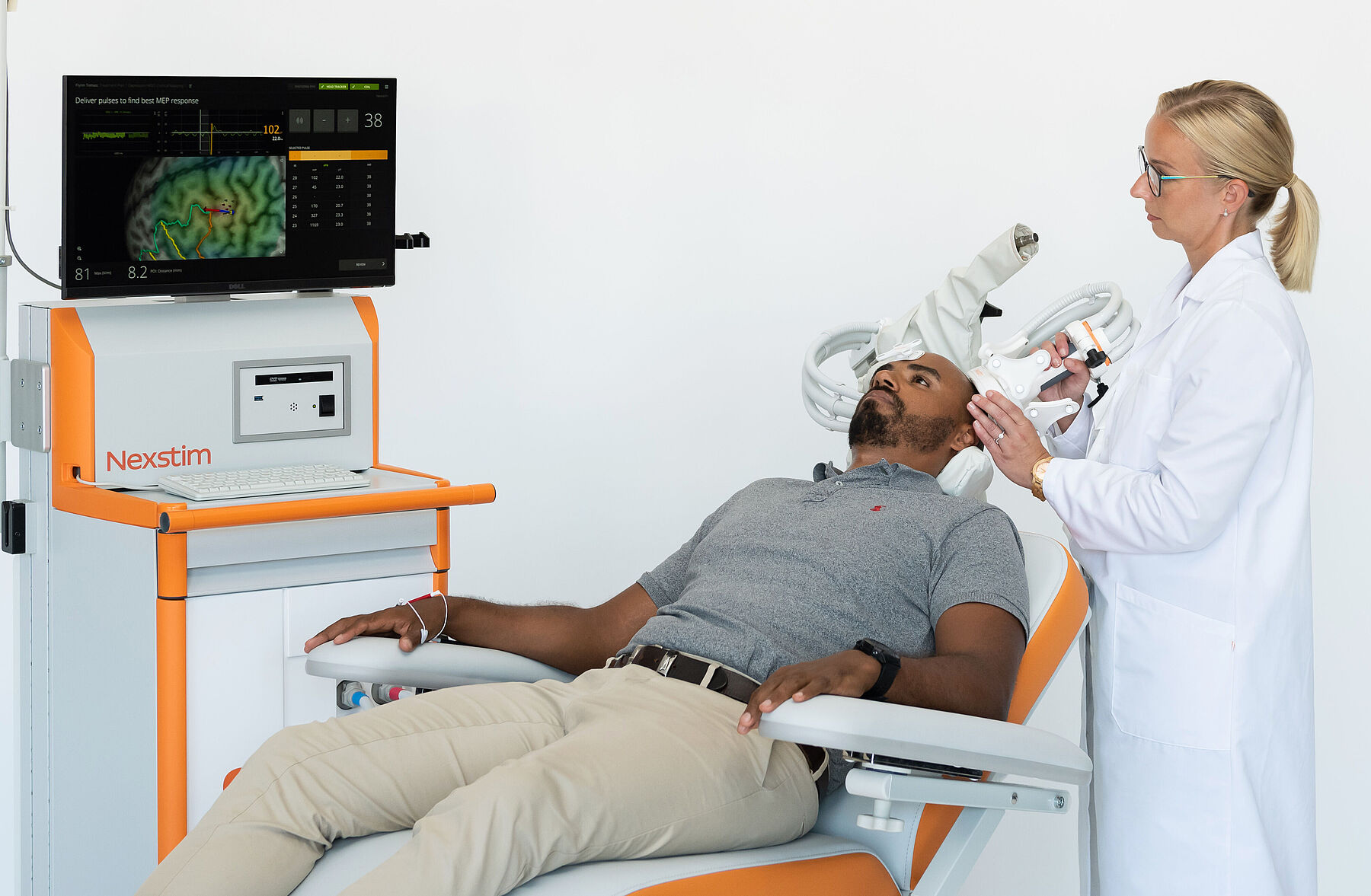
First an MRI scan of your head is taken. Your MRI scan combined with the measurements made by our SmartFocus® TMS gives a detailed image of your brain. With that, it is easy to find the exact spot where the therapy should be targeted in your brain. You can even see the target yourself on the 3D model. In addition, with SmartFocus® TMS your motor threshold is measured: it means measuring the optimal stimulation dose for you.
During therapy, an operator gently stimulates the pre-defined spot of the cortex in your brain using a wand-like TMS coil resting against your head. When the TMS coil is running, a brief magnetic field passes harmlessly through the head, activating neurons in the cortex under the coil. This procedure doesn’t cause pain, but you may feel a little tingling in the scalp.
The therapy sessions can be repeated easily and accurately, because your vital data are saved. This is how you always receive the same, prescribed dose to the same location.
Is the SmartFocus® TMS depression therapy right for me?
If you have found antidepressants aren’t working for you, SmartFocus® TMS therapy by Nexstim might be the answer. TMS has been proven safe and can be very effective with none of the drug-related side-effects. Ask your doctor about the possibility to get a SmartFocus® TMS treatment.
How long is the SmartFocus® TMS treatment?
Typical treatment course is 5 session per week for 6 weeks, or as your doctor prescribes.
After the onetime setup process, where your brain and cortex are accurately measured, each treatment session takes approximately 37 min. After a treatment session, you will be free to resume your daily routine.
Where does the SmartFocus® TMS therapy take place?
Treatment with SmartFocus® TMS will typically take place in a clinic or an office. Afterwards, you will be free to continue your daily routine.
Who performs the SmartFocus® therapy?
SmartFocus® TMS therapy is given by a trained healthcare professional, who may be a doctor, nurse, or a professionally trained TMS specialist.
What happens when I arrive?
Your physician will have assessed your medical condition and medication, to ensure there is no limitation on TMS therapy.
Before starting the Smartfocus® TMS treatment, you will have had an MRI scan of your head taken, either at you own clinic or at the hospital's radiology department. When you arrive for the first session, you will sit in a reclining seat with a comfortable headrest. Your MRI head scan is uploaded to the SmartFocus® TMS System via the hospital computer network (or from a storage medium).
What does the operator do?
Before the therapy can be started, the treatment will be personalised for you and for your brain. This means measuring your brain cortex and the stimulating dose that is optimal for you.
To measure the right stimulation dose for you, the operator will place small electrodes on the skin over your hand muscles. The electrodes are normal surface electrodes which use gel or paste. This method of detecting muscle movement is called electromyography (EMG).
In addition, you will be asked to wear a tracker on your forehead with reflecting spheres. The reflective spheres allow an infrared tracking system to follow the movement of your head and the coil in real-time.
The operator will then place a pointer against certain positions on your scalp and enter their locations into the system. This process will align your head with the 3D rendering of your head.
The operator will pick up the wand-like coil and rest it gently against your head. You will not feel any weight on your head. When the operator triggers the coil, it will induce a stimulating electric field in the underlying cortex of your brain. You will hear a click and you may feel a little tingling in your scalp. Depending on the stimulus strength, you may notice a small twitch in a muscle where the electrodes have placed.
By moving the coil and stimulating your brain several times, the operator will first find the area in your cortex from which you move your main thumb muscle. Once the optimal location has been found, the operator will determine how strong the stimuli needs to be, in order to just cause the thumb muscle to get activated. This level is your motor threshold. From this result, the operator can set the optimal stimulation power needed for comfortably and reliably conduct the depression therapy.
After that the operator will determine the exact location of the left DLPFC target based on your brain anatomy. The exact spot will be saved and so you will receive the therapy always on that exact spot.
After these measurements made in the first session, the therapy can be conducted. In the therapy session the operator opens your data from the software. He or she will place the headtracker on your forehead, but you don’t need any electrodes on your hand anymore. Then the operator can start the therapy by stimulating the previously measured spot with the stimuli personalised for you.
What do I need to do during the treatment session?
You will be asked to sit in a reclining seat with a comfortable headrest. Any kind of loose hairstyle or clothing is appropriate. Spectacles and metal earrings need to be removed.
You will be asked to wear a tracker on your forehead with reflectors. The tracker allows you to move around during the mapping - any change in your head position will be taken into account by the System's computer. In the first session the operator will also place the electrodes on your hand.
During the therapy session you will wear earplugs that the operator offers you: there is not a lot of noise but a clicking sound.
It’s important that you are having a relaxed and comfortable position during the therapy session.
What happens after the session?
Once the session is over the operator will remove the head tracker, the earplugs and any electrodes. You can leave the clinic and continue your daily routine.
Indications for use and patient safety
INDICATIONS FOR USE
CE mark and FDA clearance (K170902, K182700): Nexstim NBS 6 is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode.
NBS 6 THERAPY SHOULD NOT BE GIVEN TO
- Patients with non-removable conductive, ferromagnetic, or other magnetic-sensitive metal anywhere in the head or within 30 cm (12 in) of the stimulation coil. Examples include cochlear implants, implanted electrodes or stimulators, aneurysm clips or coils, stents, bullet fragments, ocular implants, and stents.
- Patients who have an active or inactive implanted device (including device leads), including deep brain stimulators, cochlear implants, cardiac pacemakers, and vagus nerve stimulators. Contraindicated use could result in serious injury or death.
- Patients with increased intracranial pressure or patients with intracardiac lines, intravenous pumps, or dose calculators.
Failure to follow these restrictions could result in serious injury or death.
RISKS AND SIDE EFFECTS
Seizures (convulsions): Cortical magnetic stimulation runs the risk of inducing seizures; although they are rare. Under ordinary clinical use, the estimated risk of seizure is approximately 1 in 30 000 treatments (0.003%) or 1 in 1000 patients (0.1%).
Headache: The most common side effects reported during clinical trials are mild headache (~50% of TMS treatment group) and scalp pain or discomfort (35.8%). In general, headache and pain on the stimulation site have been generally mild to moderate and occurring less frequently after the first week of treatment. The reason for headache may be the tension of scalp and neck muscles due to an uncomfortable and stressful situation.
Muscle Twitching: You may feel twitches in the muscles of your arm, leg or face during the magnetic stimulation. This is a common sensation but not hazardous. The twitches will stop when the magnetic stimulation stops.
Skin Irritation: There is a small risk of mild skin irritation at the location where the muscle electrode sensors have been placed, but this usually consists of minor redness that will go away quickly after they are removed.
Changes in hearing: The loud “click” produced by the TMS stimulator can cause temporary hearing changes following treatment. This is prevented by wearing soft foam ear plugs during treatment. No problems with hearing due to TMS have ever occurred when earplugs have been properly worn
INEFFECTIVE TREATMENT
There is no evidence that single therapy sessions would improve mood. rTMS treatment effects in reducing depression are temporary, and patients may need to continue other forms of depression therapy. Relapse into depression is likely without follow-up treatment. Notify your doctor in case of worsening depression or suicidality.
CAUTION: SPECIAL POPULATIONS
All patients must be screened for the characteristics listed in this section and excluded without clear benefit or compelling clinical reason.
The safety and effectiveness of Nexstim TMS treatment has not been established in the following patient populations:
- Younger than 22 years or older than 70 years
- Suicide plan or recent suicide attempt
- History of concurrent use of electroconvulsive therapy (ECT) or vagus nerve stimulation (VNS)
- Depression secondary to a general medical condition or substance-induced
- Seasonal affective disorder
- History of substance abuse, obsessive compulsive disorder, or post-traumatic stress disorder
- A psychotic disorder, including schizoaffective disorder, bipolar disorder, or major depression with psychotic features
- History of increased intracranial pressure or head trauma
- Cardiac pacemakers, implantable cardioverter defibrillators, ocular implants, deep brain stimulators, vagus nerve stimulators, implanted medication pumps, intracardiac lines, or significant cardiac disease
- Pregnant or nursing.


