Next-generation TMS, from Nexstim
To be an effective therapy, TMS needs to reach the part of your brain closely involved in emotion and mood. It’s quite small. Doctors know it by the name the left dorsolateral prefrontal cortex (DLPFC) and believe it is one of the most inter-connected parts of the brain. You can’t see it. In fact, no-one can, unless you have a brain scan.
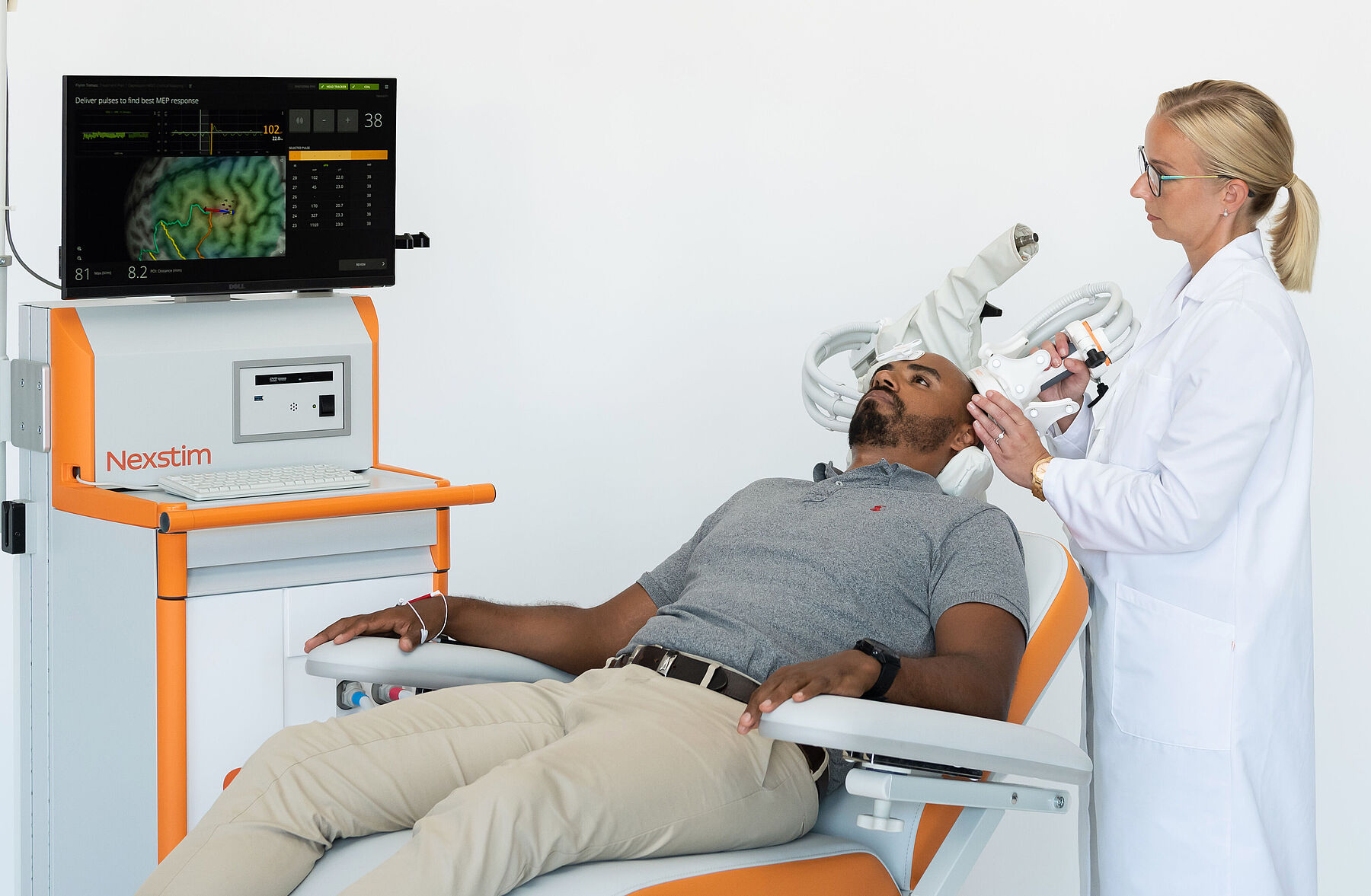
TMS used without image-based navigation simply estimates a spot for stimulation. At Nexstim, we believe it is important that TMS targets the right location in your brain – otherwise you may not get the benefit you truly need. So, we use the latest science – 3D brain imaging and proven navigation of the TMS.
Take your life back with Nexstim
Now there is a way to get the most out of every TMS treatment. All our systems utilize SmartFocus® nTMS technology, the most advanced targeting method on the market. Every treatment is personalized to your brain’s anatomy and alertness level, to offer you:
- A measured and individualized dose of TMS
- An unrivalled level of accuracy, as demanded by brain surgeons
- Treatment sessions personalized for you and your brain
- Assurance you received your prescribed dose
- Unsurpassed safety and comfort
Hear from a patient who received SmartFocus nTMS treatment
How TMS works
TMS is an application of science that allows us to communicate directly with neurons in the brain in their own language—electricity.
Download Brochure for Patients

View
Displays 3D view of your brain from MRI scan
Measure

Personalizes the therapy to your brain state
Target

Pinpoints the therapy target in your brain
Treat

See the therapy on target
- feel confident
Repeat

Your vital data are saved - ready for your next session

SmartFocus® nTMS — a new non-invasive, non-drug therapy for depression.
Indications for use and patient safety
INDICATIONS FOR USE
CE mark and FDA clearance (K170902, K182700): Nexstim NBS 6 is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode.
NBS 6 THERAPY SHOULD NOT BE GIVEN TO
- Patients with non-removable conductive, ferromagnetic, or other magnetic-sensitive metal anywhere in the head or within 30 cm (12 in) of the stimulation coil. Examples include cochlear implants, implanted electrodes or stimulators, aneurysm clips or coils, stents, bullet fragments, ocular implants, and stents.
- Patients who have an active or inactive implanted device (including device leads), including deep brain stimulators, cochlear implants, cardiac pacemakers, and vagus nerve stimulators. Contraindicated use could result in serious injury or death.
- Patients with increased intracranial pressure or patients with intracardiac lines, intravenous pumps, or dose calculators.
Failure to follow these restrictions could result in serious injury or death.
RISKS AND SIDE EFFECTS
Seizures (convulsions): Cortical magnetic stimulation runs the risk of inducing seizures; although they are rare. Under ordinary clinical use, the estimated risk of seizure is approximately 1 in 30 000 treatments (0.003%) or 1 in 1000 patients (0.1%).
Headache: The most common side effects reported during clinical trials are mild headache (~50% of TMS treatment group) and scalp pain or discomfort (35.8%). In general, headache and pain on the stimulation site have been generally mild to moderate and occurring less frequently after the first week of treatment. The reason for headache may be the tension of scalp and neck muscles due to an uncomfortable and stressful situation.
Muscle Twitching: You may feel twitches in the muscles of your arm, leg or face during the magnetic stimulation. This is a common sensation but not hazardous. The twitches will stop when the magnetic stimulation stops.
Skin Irritation: There is a small risk of mild skin irritation at the location where the muscle electrode sensors have been placed, but this usually consists of minor redness that will go away quickly after they are removed.
Changes in hearing: The loud “click” produced by the TMS stimulator can cause temporary hearing changes following treatment. This is prevented by wearing soft foam ear plugs during treatment. No problems with hearing due to TMS have ever occurred when earplugs have been properly worn
INEFFECTIVE TREATMENT
There is no evidence that single therapy sessions would improve mood. rTMS treatment effects in reducing depression are temporary, and patients may need to continue other forms of depression therapy. Relapse into depression is likely without follow-up treatment. Notify your doctor in case of worsening depression or suicidality.
CAUTION: SPECIAL POPULATIONS
All patients must be screened for the characteristics listed in this section and excluded without clear benefit or compelling clinical reason.
The safety and effectiveness of Nexstim TMS treatment has not been established in the following patient populations:
- Younger than 22 years or older than 70 years
- Suicide plan or recent suicide attempt
- History of concurrent use of electroconvulsive therapy (ECT) or vagus nerve stimulation (VNS)
- Depression secondary to a general medical condition or substance-induced
- Seasonal affective disorder
- History of substance abuse, obsessive compulsive disorder, or post-traumatic stress disorder
- A psychotic disorder, including schizoaffective disorder, bipolar disorder, or major depression with psychotic features
- History of increased intracranial pressure or head trauma
- Cardiac pacemakers, implantable cardioverter defibrillators, ocular implants, deep brain stimulators, vagus nerve stimulators, implanted medication pumps, intracardiac lines, or significant cardiac disease
- Pregnant or nursing.


